Selection, access and use of in vitro diagnostics
Access to good quality, affordable, and appropriate health products is indispensable to advance universal health coverage, address health emergencies, and promote healthier populations. Without access to In vitro diagnostics (IVDs), health providers cannot diagnose patients effectively and promptly or provide appropriate treatments.
HIV screening test in Pakistan A lab technician conducts HIV screening test at Hayatabad medical complex, Peshawar district.
The WHO Essential Diagnostic List (EDL) aims to provide evidence-based guidance and set a reference for the development or update of national lists of essential in vitro diagnostic tests. National essential medicines lists have been successful facilitating access to affordable medicines, particularly in low-resourced countries. It is expected that national essential diagnostics lists will provide similar benefits and improve access to essential in vitro diagnostic tests. It will also contribute towards health systems strengthening and realizing universal health coverage, which is central to Goal 3 of the Sustainable Development Goals (Ensure healthy lives and promote well-being for all at all ages).
What is the WHO Essential Diagnostic List (EDL)?
WHO model list of essential in vitro diagnostics (EDL)
Open calls
There are no open calls.
Closed calls
Events
Publications
The selection and use of essential in vitro diagnostics: report of the fourth meeting...
Selection of essential in vitro diagnostics at country level: using the WHO Model List of Essential In...
This document intends to provide guidance to countries on methods for developing and updating national lists of essential in vitro diagnostics (NEDL)....
Report of the third meeting of the WHO Strategic Advisory Group of Experts on In Vitro Diagnostics, 2020 (including the third WHO model list of essential...
The selection and use of essential in vitro diagnostics: WHO technical report series;1022
The report of the second meeting of the Strategic Advisory Group of Experts on In Vitro was launched in November 2019 and includes the updated WHO Model...
The electronic WHO model list of essential in vitro diagnostics (eEDL)
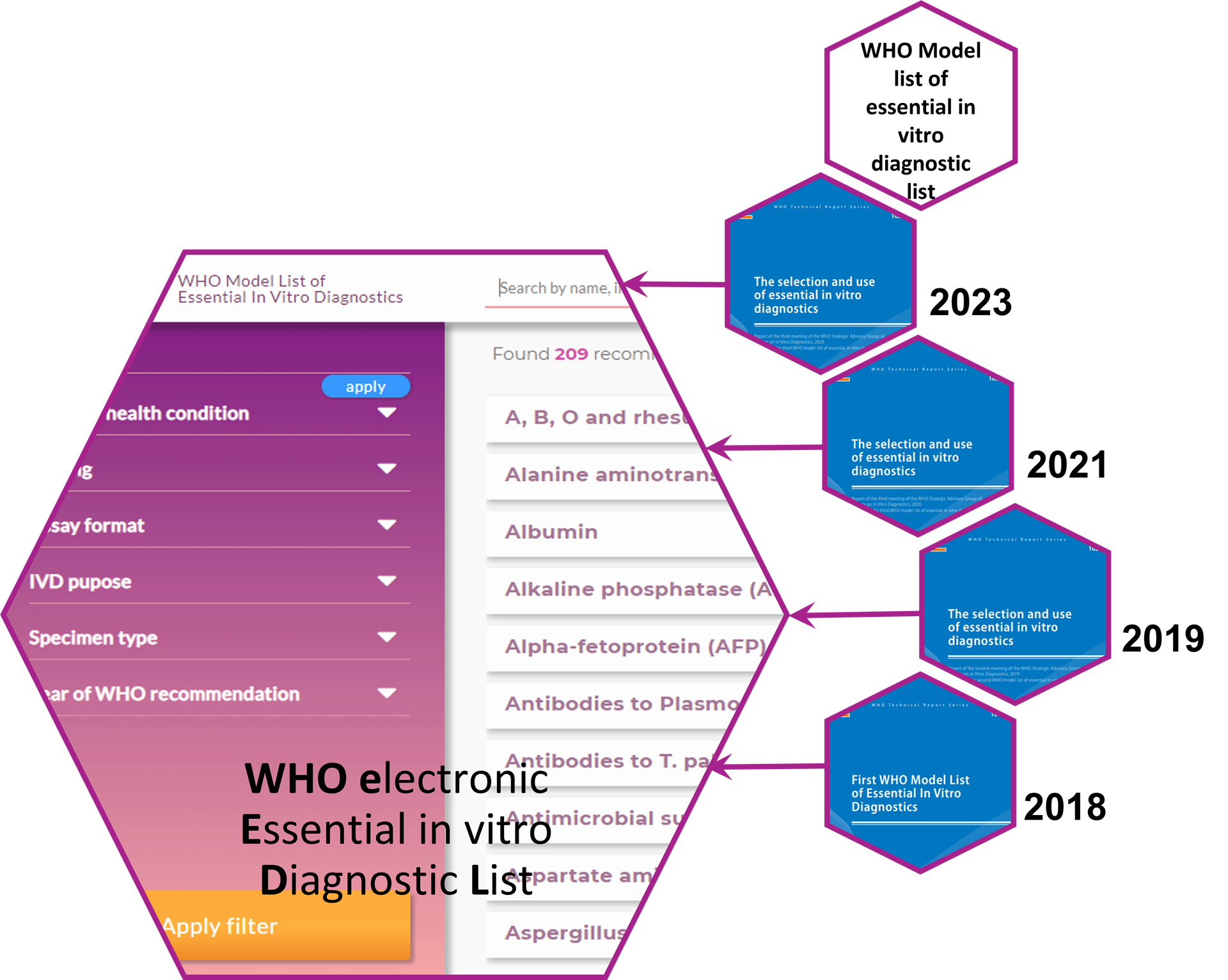
What is the eEDL?
The eEDL (electronic essential in vitro diagnostic list) is an open access electronic database of in vitro diagnostics. The eEDL compiles the diagnostics that appear in the WHO model list of essential in vitro diagnostics.
The eEDL and other WHO electronic health products lists
WHO has developed a series of open access electronic databases to support the scale-up of Universal Heath Coverage (UHC). The lists aim to increase access to health products for intervention delivery packages. Electronic databases ensure access to the latest information since they can be updated and expanded. The electronic intervention and health products lists for UHC include:

2025 Strategic Advisory Group of Experts on In Vitro Diagnostics (SAGE IVD)
The World Health Organization (WHO) has established a Strategic Advisory Group of Experts on In Vitro Diagnostics (SAGE IVD) to act as an advisory body on matters of global policies and strategies related to in vitro diagnostics (IVDs). The SAGE IVD is expected to meet every two years to make recommendations on the content, format, and implementation of the EDL and on any matters related to IVDs.
Related Health Topics
Related Teams
Contact us
For comments or input on the EDL please contact the secretariat at:



